Recent advances in marine ornamental breeding and seed production at Vizhinjam Regional Centre of CMFRI India
- 1The Indian Council of Agricultural Research (ICAR)-Vizhinjam Regional Centre of CMFRI, Thiruvananthapuram, Kerala, India
- 2Thangal Kunju Musaliar (TKM) College of Arts and Science, Kollam, Kerala, India
- 3Central Marine Fisheries Research Institute (CMFRI), Kochi, Kerala, India
The marine ornamental fish trading industry is an ever-expanding one, as indicated by its bolstering statistics, which amount to about US$ 300 million. Although the trade is booming, the natural habitats that foster these fishes, such as coral reefs, are steadily declining. One of the reasons for this is the overdependency of the industry on wild-caught fishes. To surpass this, the Central Marine Fisheries Research Institute (CMFRI) has developed hatchery technology for breeding marine ornamental fishes such as seahorses, clownfishes, damsels, and serranids. It began with the breeding of Hippocampus kuda. This progressed to breeding clownfishes such as Amphiprion chrysogaster, A. ocellaris, A. nigripes, A. peridarion, and A. ephippium and Premnas biaculeatus. Damsel fishes that were bred successfully include Chrysiptera cyanea, C. hemicyanea Neopomacentrus cyanomos, N. nemurus, and Dascyllus carneus. The recirculating aquaculture system (RAS) designed in the Vizhinjam Regional Centre of CMFRI is being used for broodstock development of serranids, tangs, squirrel fishes, and soldier fishes. A standard method was developed for captive breeding and hatchery rearing of anthias Pseudanthias marcia and P. squamipinnis in an advanced indigenous RAS system. The present article provides a bird’s eye view of the important research work done in India concerning marine ornamental fish breeding and reviews important breeding work carried out at Vizhinjam Regional Centre of CMFRI.
Introduction
The marine ornamental fish industry depends mainly on the organisms harvested from the wild (Wabnitz et al., 2003). This demand has created immense pressure on the natural stocks of fish. If the harvesting of fishes from the wild must stop, new modes to culture these fishes must emerge. The only solution to this problem is aquaculture, which could provide a growing proportion of marine ornamental fish in a very short time (Molina and Segade, 2012). Out of the 1,000 species of coral reef fish traded (Green, 2003), only 51 have been cultured in captivity for the aquarium trade (Arvedlund et al., 2000) and very few in commercial quantities. Approximately 30 million marine reef fish belonging to roughly 1,800 different species are commercialised every year worldwide (Rhyne et al., 2012; Thornhill, 2012, and Wabnitz et al., 2003).
Family Pomacentridae is important in the marine ornamental fish trade because it accounts for the second-largest marine ornamental fish import into the United States (Rhyne et al., 2012). Since these fishes have greater demand in the aquarium trade, attempts have been made to breed them successfully. Pomacentrids are continuous spawners and easily lay eggs if provided with the proper water quality conditions and food, the only contradiction to this being members of the genus Abudefduf. Even though reports of successful breeding of Pomacentrids of genera Amphiprion and Premnas have been published, successful larval rearing of damselfishes is still in its infancy.
There are only a few reports regarding the successful larval development of many damselfish, such as Abudefduf saxatilis (Alshuth et al., 1998 and Wittenrich et al., 2012), Microspathodon chrysurus (Potthoff et al., 1987), and Pomacentrus amboinensis (Murphy et al., 2007). Breeding and larviculture of the sapphire devil damselfish Chrysiptera cyanea were carried out by Gopakumar et al. (2009). Breeding and larval rearing of the damselfishes such as N. filamentosus, N. nemurus, and P. caeruleus under captivity were also studied by Gopakumar et al. (2002). Breeding, early development, and larval rearing of cloudy damsel Dascyllus carneus were carried out by Anzeer et al. (2019). Santhosh et al. (2021) have developed captive breeding protocol for Azure damsel.
In recent years, a plethora of breeding programmes have been launched for breeding many pomacentrid species, most notably in clownfishes. Breeding of A. chrysogaster (Gopakumar et al., 1999), A. sebae (Ignatius et al., 2001), A. ocellaris (Ajith kumar and Balasubramanian, 2009 and Madhu et al., 2012), A. percula (Madhu and Rema, 2011), Premnas biaculeatus (Madhu et al., 2006 and Anil et al., 2010), A. frenatus (Madhu and Rema, 2010), A. nigripes (Anil et al., 2012), A. akallopisos (Dhaneesh et al., 2012), A. ephippium (Rohini Krishna et al., 2018), and Black A. ocellaris (Raheem et al., 2021) have been carried out successfully. Thomas et al. (2015) studied the spawning behaviour and embryonic development of sebae anemone fish A. sebae (Bleeker, 1853) in captivity.
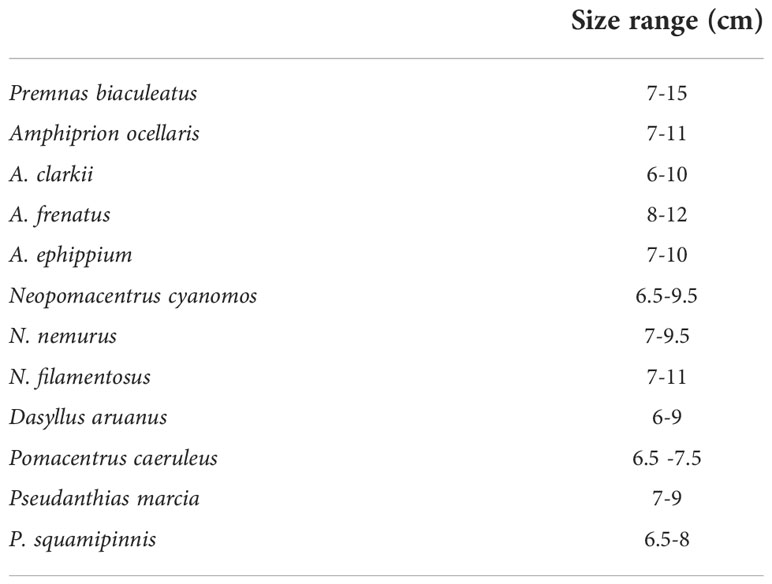
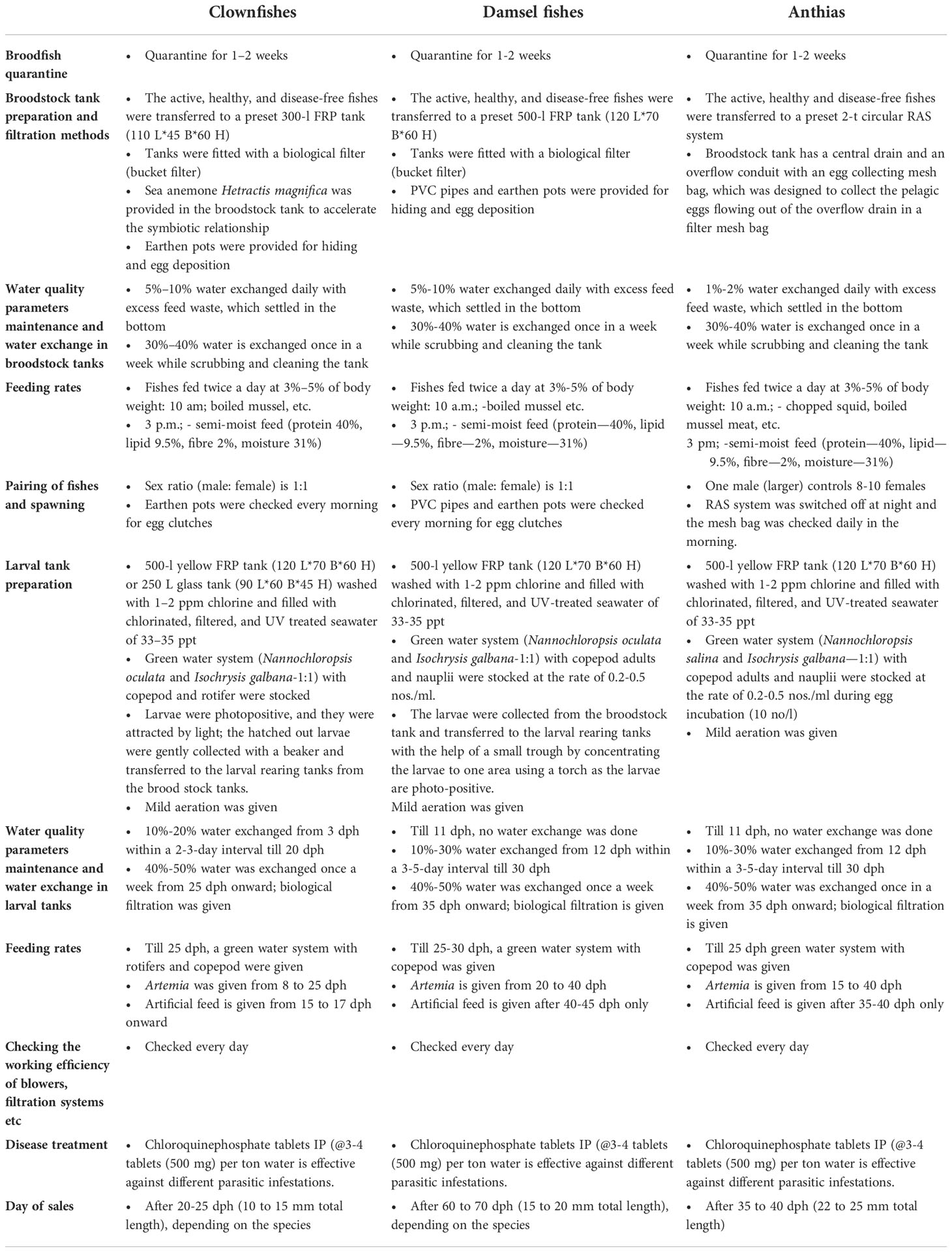
Although some of these programmes have tasted success, the detailed information on the embryonic development and standardised protocol for larval rearing many species is very meagre. Broodstock collection, maintenance, spawning, embryonic development, and larval growth and development of clownfishes, damsels, and anthias were detailed in the present article. The species bred successfully at the centre were clownfishes Amphiprion ephippium, popularly known as red saddleback anemonefish; A. clarkii, popularly known as Clark’s anemone fish; A. frenatus known as tomato clownfish; A. ocellaris known as false percula clownfish; Premnas biaculeatus known as spine-cheeked anemonefish; damsel fishes Neopomacentrus cyanomos, N. nemurus, Dascyllus carneus, D. aruanus, and Chrysiptera hemicyanea; and vibrant multicoloured Anthid fishes which come under the family Serranidae such as Pseudanthias marcia (Marcia’s anthias) and sea goldie, P. squamipinnis. The summary is given in Table 1.
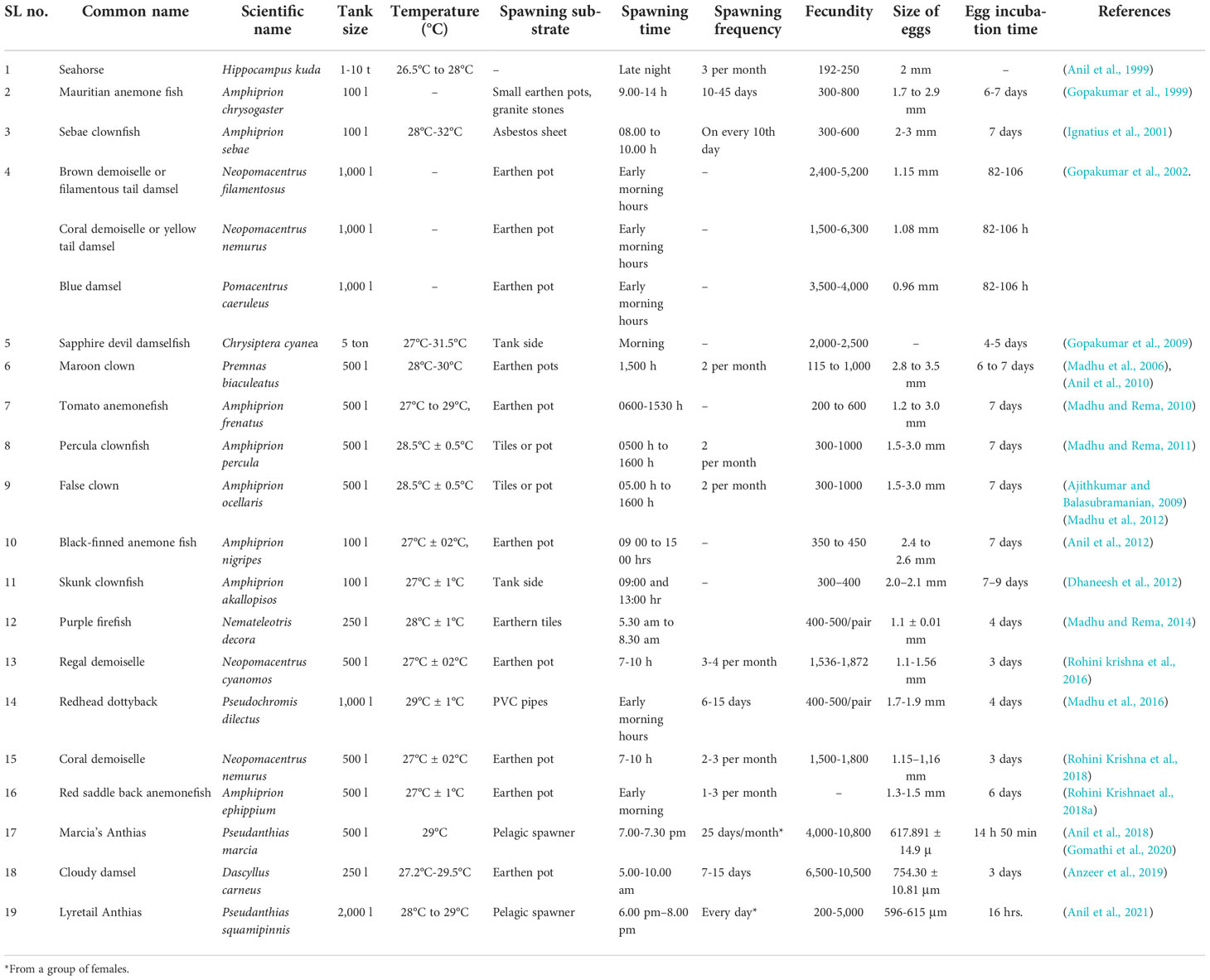
Table 1 Summary of natural spawning, environmental conditions, and reproductive features of marine ornamental fish carried out in India.
Brood stock collection, development, and maintenance
Sexually mature/subadults fishes of clownfish, damsel fish, and anthias were collected from the wild/ornamental traders and the size range of brooders is given in Table 2. The size range of maturity of the fishes may vary slightly depending on the environmental conditions.
Broodstock tanks
The clownfishes and damselfishes were usually stocked in 250–500-l tanks. Clownfishes could also be stocked in multi-tank RAS with several units made up of fibreglass with an acrylic front panel of 200-l capacity each (Figure 1). A single recirculatory filtration system for a chain of tanks worked efficiently for the broodstock maintenance and spawning of clownfishes. Damsel fishes were stocked in rectangular FRP tanks of 500-l capacity (Figure 1). Each species was given a separate broodstock tank. The tanks were fitted with biological filters to maintain the water quality. Uneaten food was siphoned out twice daily. The siphoned-out water was then replaced with fresh seawater. In addition to this, 25% of the culture water was exchanged with fresh seawater once a week. During the rearing period, water quality parameters such as water temperature, pH, salinity, NH3, nitrate, and phosphate contents were measured using standard methods. Salinity and pH are important parameters demanding careful monitoring. Salinity was maintained in the range of 32–34 ppt and pH 8–8.2. The temperature of the water was maintained at 27 ± 02°C. The fish were fed four times a day or as 5% of the body weight or as ad libitum. The feeding schedule followed was pellet feed at 10:00 a.m., boiled mussel meat at 12:00 p.m. and 2:00 p.m., and Artemia nauplii at 4:00 p.m. A group of fishes were kept together in either RAS units or FRP tanks; once they showed pairing behaviour and started behaving aggressively to the other fishes, the other fishes were moved to separate broodstock tanks. In the case of clownfishes, once pairing is over, other fishes are removed, isolating the pair, whereas in the case of damsels, up to five to six fishes can be kept in the broodstock tank with multiple fishes spawning in the tank. The fishes were provided with earthen pots or tiles for laying eggs.
Anthias brood fishes were kept in recirculatory aquaculture systems (RAS). After a quarantine period of 2 weeks, the active, healthy, and disease-free juvenile fishes were transferred to 2-t FRP tanks set with RAS (Figure 2). The broodstock RAS tank is a part of a multi-tank system provided with a common filtration designed for broodstock development of tangs, anthias, squirrel fishes, and soldier fishes which are also being attempted for breeding. Each broodstock tank has a central drain and an overflow conduit (side drain). Around 1%–2% of the water was changed daily along with waste settled at the bottom. The water which is incoming gets circulated, and part of it passes out through the central drain and goes to the settling tank, where the waste gets collected. The water overflowing from the settling tank will then pass through the biological filter unit into the reservoir. Water which goes out through the side drain also gets collected in the reservoir tank. Water from the reservoir is then pumped to the protein skimmer connected to the system, a part of which will then get distributed to all the five brood stock tanks after passing through a UV filter. The central drain valve regulates the percentage of water going out through the central drain and the side drain. Water quality parameters of the broodstock system such as water temperature, salinity, pH, ammonia, and nitrate were checked weekly and recorded. A natural photoperiod of 12 h L: 12 h D was maintained throughout the culture period weekly, once 5%–10% water was exchanged after scrubbing and cleaning the tank.
It is easy to collect eggs in the RAS system in case of fishes like anthias having pelagic floating eggs, and in the case of fishes like damsel and clownfish which have attached demersal eggs, they are either allowed to hatch in the tank itself or can be taken out and hatched on the final day of the incubation period. The eggs from the pelagic spawners are collected by tying a net sock or keeping a sieve of 200 µm which is kept immersed in water to prevent the drying of eggs.
Physicochemical parameters
Physicochemical parameters, such as salinity, pH, dissolved oxygen, water temperature, and NH3, were checked twice a week using standard methods. Water quality parameters of the broodstock system during the breeding period were as follows: temperature: 29 ± 1°C, salinity: 33–35 g/l, pH: 7.6–8, total ammonia: 0.354–0.512 mg/l, D.O: 4.7–5.6 mg/l, alkalinity: 100–130 mg/l CaCO3, CO2: 4.15–4.80 mg/l; turbidity: 0.16–0.39 NTU, nitrate (NO3): 0.3–0.7 mg/l, nitrite (NO2): 0.007–0.029 mg/l; and photoperiod: 12 h L: 12 h D.
Live feed culture
Live feeds such as phytoplankton, copepods, Artemia, and rotifers were mass cultured for larval rearing. For feeding zooplankton culture and for maintaining green water in the rearing tanks, the stock cultures of algae, viz, Nannochloropsis oculata and Isochrysis galbana, were maintained in a stock culture room at 24°C in 250-ml flasks and 3–4-l flasks. Then, the cultures were upscaled to 20-l carboys and 200-l translucent plastic tanks.
Rotifer culture
Rotifers are the commonest live feed species used in hatchery rearing of ornamental fish larvae, apart from artemia and copepods. Rotifers belonging to the genus Brachionus are commonly used in aquaculture. For the breeding of marine fishes, the rotifers of different species such as B. plicatilis and B. rotundiformis are commonly used in the early larval stages. Rotifers are grown in mass quantities for aquaculture because of their rapid reproduction, have the ability to grow in high density, have tolerance to wide temperature and salinity variations, and are readily accepted by larval fishes. Two prominent stains of B. plicatilis are used in hatcheries: (1) small strain (S type with average lorica length of 130 µ) and (2) large type (L type with average lorica length of 240 µ). Both strains show different temperature and salinity tolerances. The choice of rotifer differs in accordance with the mouth size of the larvae. For an ornamental hatchery, rotifers can be cultured in small glass tanks of 250-l capacity. To get a good yield, water quality parameters should be at the optimum level: the temperature at 25°C, pH at 7.5–8.5, salinity in a 20–30-ppt range, and less than 1 mg/l free ammonia (NH3). Stock culture should be maintained separately. Rotifers can be fed with different microalgal species such as Nannochloropsis spp. or I. galbana. The microalgae can be cultured on site, or if the algal culture facility is not available, rotifers can also be fed with algal paste, condensed algae, or rotifer feed which are available in the market. Aerating the rotifer culture is essential to providing oxygen, keeping rotifers and food cells suspended, and optimising tank cleanliness. Although rotifers are less nutritionally rich than copepods, their nutritional composition can be adjusted relatively quickly by the enrichment method.
Artemia culture
The hatching of cysts was carried out in semitransparent open cylindrical tanks with aeration in filtered seawater. When the aeration is removed before harvesting, the unhatched cyst floats on the top and the nauplii concentrates at the bottom of the tank. The concentration of nauplii at the bottom was increased by shading the upper part of the tank and focusing a light source on a small area at the bottom. When the nauplii had settled in the bottom, they were siphoned off within 5–10 min. The siphoned-off water passed through a filter of mesh size 140 µm, which was kept submerged in seawater contained in a bucket. The submersion of the filter was done to prevent physical damage to the nauplii that could happen at the time of filtration of siphoned water.
Clownfish
Parental care and spawning
The male parent exhibited a high degree of parental care for the eggs, such as fanning the eggs with the pectoral and caudal fins and removing the infected eggs with their mouth. In Amphiprion clarkii, A. frenatus, A. ocellaris, A. ephippium, and Premnas biaculeatus, spawning took place in the morning hours, usually between 6.00 and 9.00 a.m. The eggs are found attached to the substratum, and they are capsule-shaped.
The spawning frequency was found to be one to three times per month in A. clarkii, A. ephippium, A. ocellaris, P. biaculeatus, and A. frenatus. It was reported to be 2 per month in P. biaculeatus. A. ocellaris exhibited the highest spawning interval (10–32 days), followed by P. biaculeatus (11–29), A. frenatus (11–26), A. ephippium (11–22), and A. clarkii (9–13 days). The most common time interval for spawning was 11 days (A. clarkii, A. ephippium, P. biaculeatus) followed by 12 days in A. ocellaris and 15 in A. frenatus.
Embryonic development and hatching
In A. ephippium, the eggs measured 1,350–1,541 µm in length and 535–717 µm in width. In A. ocellaris, the eggs measured 1,247–1,392 µm in length and 508–560 µm in width. In A. frenatus, the eggs measured about 1,600–1,659 µm in length and 720–729 µm in width. In P. biaculeatus, the eggs measured 1,400–1,411 µm in length and 800–835 µm in width. The eggs of A. clarkii measured 1,700.6–2,300.5 µm in length and 750.5–900 µm in width.
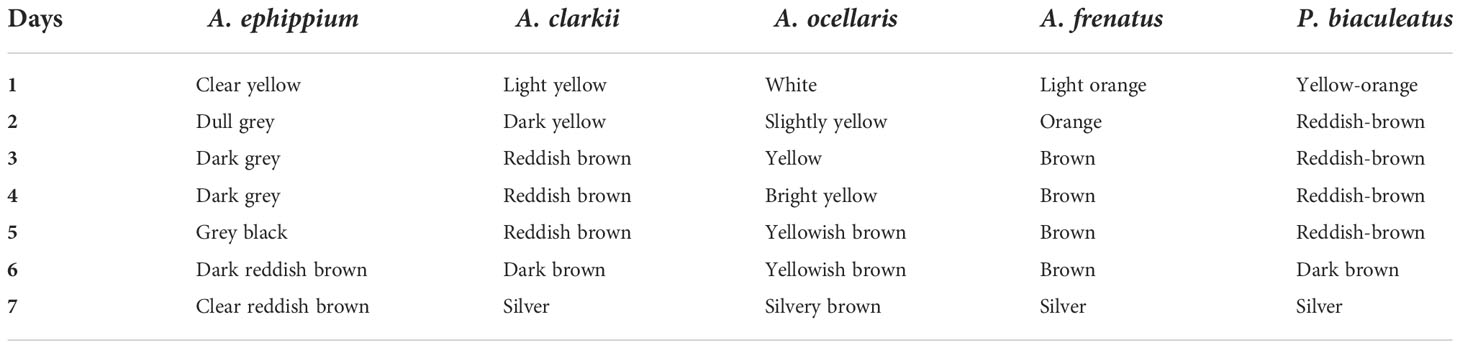
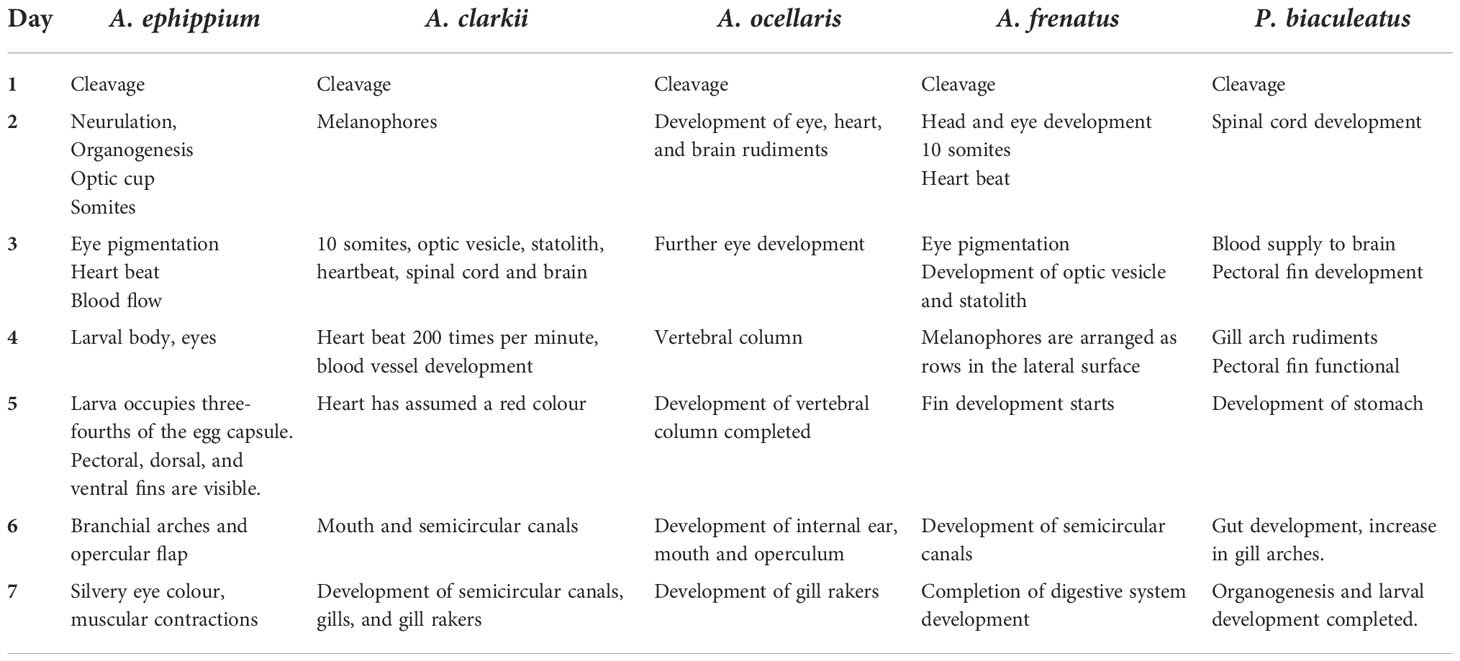
The eggs hatched on the seventh day of incubation at 28°C–30°C. Hatching took place in the early evening hours at about 7.00–8.00 p.m. The hatching occurred between 8.30 and 11.00 p.m. in A. ocellaris and 6.00 to 11.00 p.m. in A. frenatus. Since the larvae are photopositive, they were attracted by a torch and transferred to a small trough and then transferred to the larval rearing tanks. The changes in colour and embryological changes during egg development are shown in Tables 3, 4. The broodstocks of different clownfishes are depicted in Figure 3. The egg development of A. ephippium is shown in Figure 4.
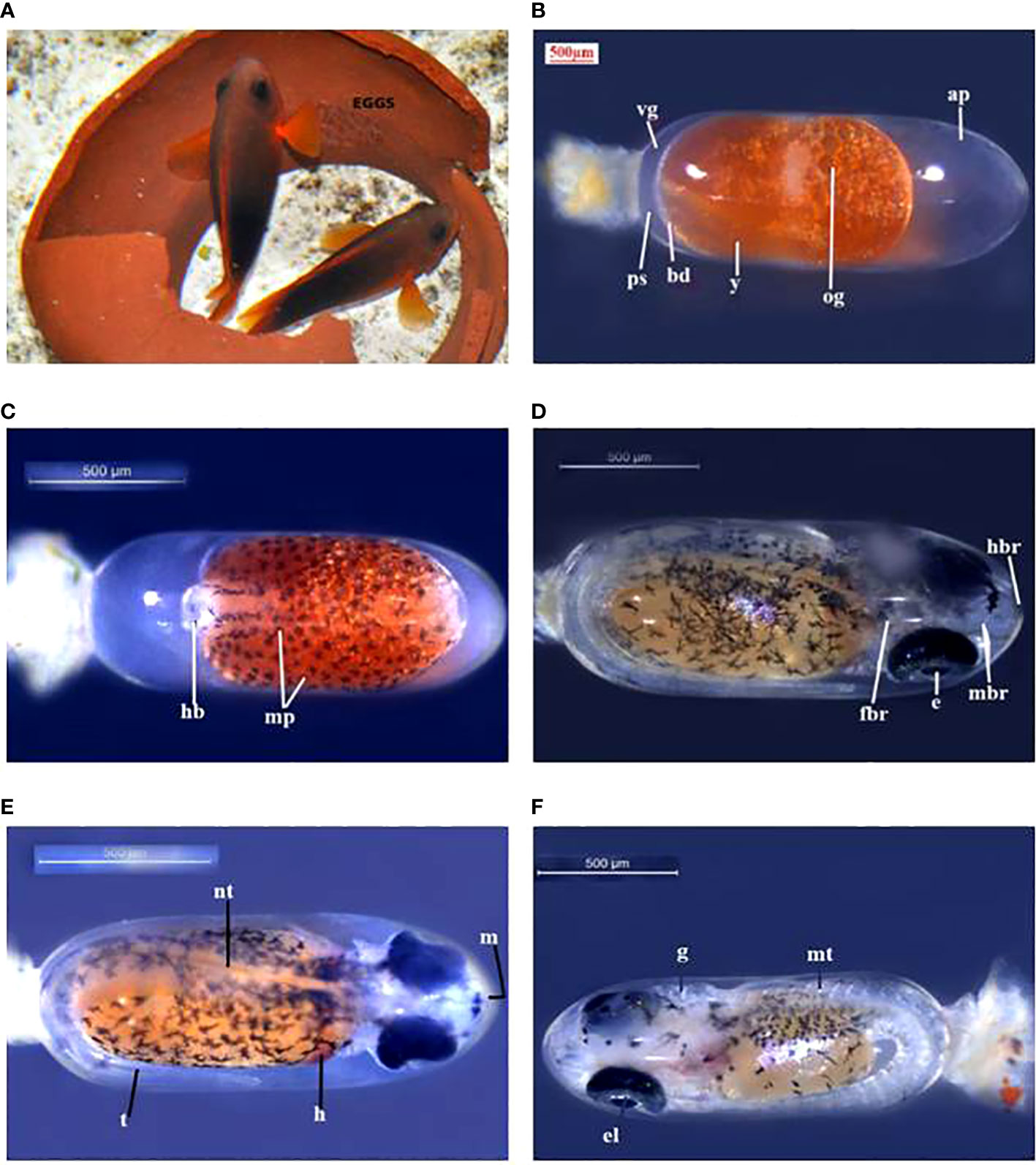
Figure 4 A. ephippium (A) broodstock, (B) egg (day 1), (C) egg (day 2), (D) egg (day 3), (E) egg (day 4), (F) egg (day 5). ap, animal pole; bd, blastodisc; e, eye; el, eye lens; fbr, forebrain; g, gills; h, heart; hb, head bud; hbr, hindbrain; m, mouth; mbr, midbrain; mp, melanophore; mt, myotomes; nt, notochord.; og, oil globule; ps, perivitelline space; t, tail; vg, vegetal pole; y, yolk.
Larval rearing and feeding schedule
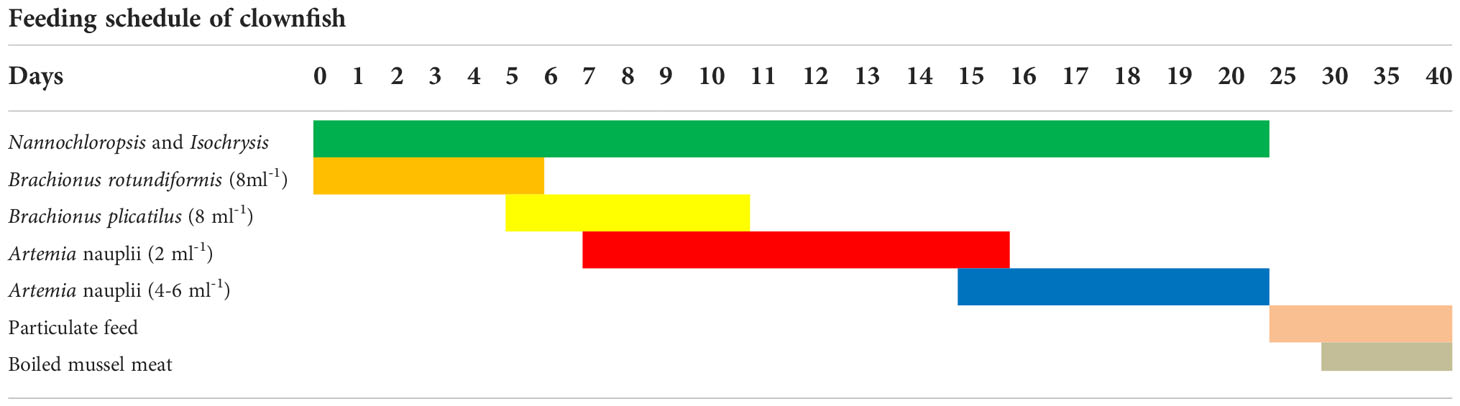
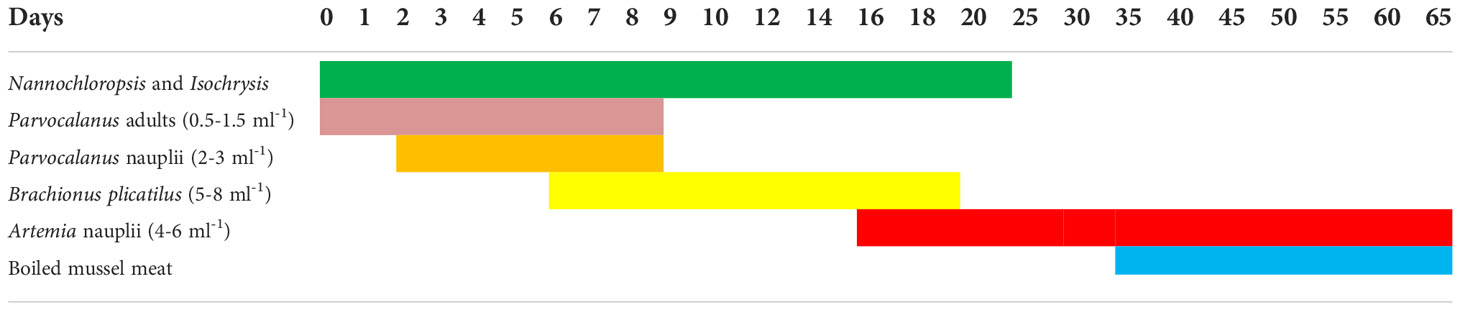
FRP tanks are used for larval rearing, and an algal density of 1 × 105–3 × 105 cells ml-1 was maintained in the tanks. All the tanks were supplied with the algae N. oculata and I. galbana (3:1) up to the 20th day. The larvae were fed on rotifers from the 1st to the 10th day of hatch. From the 1st to the 5th day, B. rotundiformis was given. From the 5th to 10th days, the rotifer given was B. plicatilis at the rate of 8 nos. ml-1. Artemia nauplii were given at the rate of 2 nos. ml-1 from the 7th day from the day of hatch, and this was slowly increased to 4 and 6 nos ml-1 up to 15th and 20th dph, respectively. Particulate feed was started from the 25th day onward using ground pellet fish feed of sizes ranging from 200 to 500 µ. They were given boiled mussel meat from the 30th day onward. Fishes were fed ad libitum. The feeding schedule of the clownfishes is given in Table 5.
The total body length of hatchlings was 4.0–4.3 mm in A. clarkii, 4.7–5.5 mm in A. ephippium, 3.8–4.5 mm in A. ocellaris, 3.9–4.2 mm in A. frenatus, and 3.8–4.2 mm in P. biaculeatus, and A. ephippium showed adult colouration early in development, i.e., when the larva was about 3 months old, while in A. ocellaris and A. clarkii, adult colouration was attained after 3.5 months of development. In A. frenatus, adult colouration was reached in about 4 months. A. clarkii appears to be the fastest-growing species in terms of both total length and body width, and A. ocellaris was the least growing.
Damsel fish
Parental care and spawning
The fishes exhibited territorial behaviour and were observed to attack other fishes if they came close to their eggs. The fishes preferred to lay the eggs on the sides of the tank. They were also seen depositing eggs on earthen pots and PVC pipes. In N. cyanomos, the fishes preferred to lay the eggs on PVC pipes other than the earthen pots or sides of the tank. N. nemurus and N. cyanomos are sexually monomorphic, and the male parent exhibited a high degree of parental care for the eggs, such as fanning the eggs with the pectoral and caudal fins and removing the infected eggs with the mouth. The fishes were also in the habit of consuming the clutch of eggs if it was disturbed.
The fishes exhibited a high spawning frequency with an average of 2–4 per month. The lowest spawning frequency was observed during May and June. The time interval between the spawning ranged from 5 to 14 days. In N. cyanomos, the fishes exhibited a high spawning frequency with an average of 1–4 per month. The lowest spawning frequency was observed during April–June and September. The highest spawning frequency was observed during January and July. The time interval between the spawning ranged from 4 to 10 days.
Embryonic development and hatching
The fertilised eggs were transparent, capsule-shaped, and demersal and contained oil globules. The eggs measured 1,156–1,168 µm in length and 380–400 µm in width. The yolk length was found to be 725 µm on the first day after spawning. The egg clutches contained 1,500–1,800 eggs with an average of 1,653.36 ± 133.55 per spawn. In N. cyanomos, the eggs measured 1.1–1.56 mm in length and 0.5–0.546 mm in width. The yolk length was found to be 906 µm and the yolk width 441 µm on the first day after spawning. The egg clutches contained 1,536–1,872 eggs with an average of 1,550.36 ± 123.55 per spawn.
Eggs were encased in a transparent capsule, the colour of which changed from transparent to yellowish in the case of spoilage. Spoiled eggs also exhibited a cloudy appearance. The egg capsule did not exhibit any external morphological alterations during development. In addition to the yolk, the eggs were provided with oil globules which equipped them with capacity for buoyancy and enabled them to stand upright while being attached to the substratum. The number of oil droplets varied from one to several. On the second day, rudiments of the head fold and tail fold were visible in the embryo. The larval body showed clear signs of development with the appearance of melanophores over the tail region. The yolk had shrunk to half of its normal size. In N. cyanomos, on the second day, the optic vesicle and tail were visible. Eye pigmentation had not yet begun.
Hatching usually occurs on the 3rd day of incubation. Hatching took place in the early evening at about 19.00–19.30 h. The hatching rate was 97%. On the 3rd day at the time of hatching, the larval body was almost fully formed. The eyes showed a bluish-silver colour, the whole body was curved up, and as a result, the tail touched the head region. These two signs indicated that the larvae were about to hatch. The larva had developed an efficient circulatory system which was evident by the heartbeat. The larval body showed signs of segmentation which was evident by the appearance of paired myomeres. The yolk had shrunken and now occupied one-third of the egg. The larval body now occupies the whole of the capsule and then breaks it off by vigorous wriggling movements at the end of the incubation period.
Larval rearing and feeding schedule
The larvae were collected from the broodstock tank and transferred to the larval rearing tanks with the help of a small trough by concentrating the larvae in one area using a torch as the larvae are photo-positive.
N. cyanomos and N. nemurus were fed with the copepod Parvocalanus crassirostris adults and nauplii from days 2 to 8 and from the 6 to 18th days onward rotifer B. plicatilis (5–8 per ml), and then from the 16th day onward co-feeding started with Artemia nauplii. Boiled mussel meat was given to the larvae from the 35th day ahead.
In damselfishes, the presence of P. crassirostris adults ensured that the smallest possible live stages of copepods nauplii were available to the newly hatched larvae and the larvae also appeared to favour the sides of the tank where the copepods were concentrated. The microscopic observation of the dissected stomach on the 4th day revealed the presence of copepod nauplii. A feeding schedule of hatchery-reared N. nemurus and N. cyanomos is given in Table 6.
The larvae were transparent and measured 2–2.4 mm in total length at the time of hatching. Since the larva was altricial and was provided with a small but regressed yolk sac, it showed that the early developmental requirements were being met by the yolk sac. Although it had a mouth gap of about 237.12 μm, the larvae did not feed on copepod nauplii immediately after hatching; however, it was found to feed on the algae provided in the tank, and the dissection of the larval body helped to confirm the same. The first nauplii of copepods are in the range of 50–100 μm, which makes it a suitable food for the damsel fish larvae. The eye was well developed in the larvae and was found protruding from the two sides of the head, which was an indication of its carnivorous and voracious habit. The embryonic and larval development of blue damsel Pomacentrus caeruleus is shown in Figure 5.
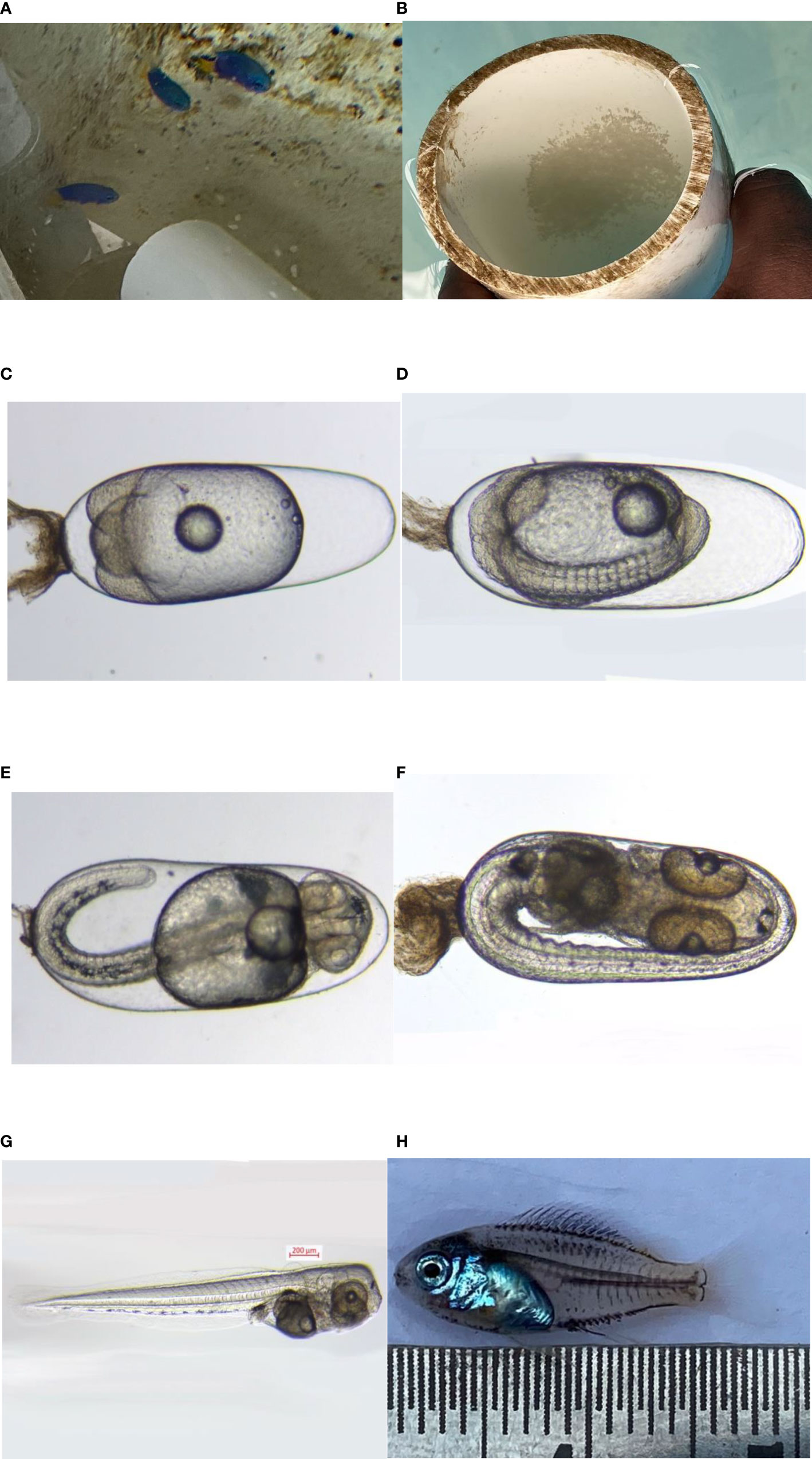
Figure 5 (A) P. caeruleus broodstock, (B) egg in substratum, (C) egg (day 1), (D) egg (day 2), (E) egg (day 3), (F) egg (day 4), (G) newly hatched larva, and (H) juvenile 27 DPH.
Anthias
Parental care and spawning
In Anthid fishes, particularly genus Pseudanthias are sexually dichromatic and dimorphic protogynous hermaphrodites. Males are larger and brightly coloured than females. Broodstocks of P. marcia and P. squamipinnis were developed in a 2-t recirculation aquaculture system (RAS) using wild-caught juveniles (Figure 6). In captivity after a period of 6–7 months of rearing, fishes started showing courtship behaviour (Anil et al., 2018). Although two males were present in the broodstock tank of P. marcia during spawning, only one active male with a bright colour exhibited darting movement around the group of females, chasing and defending its territory aggressively, without permitting the other male to enter its territory. These fishes follow a haremic reproductive system; usually one large, brightly coloured territorial male controls a group of females in the adult and subadult stages.
Eggs of P. marcia and P. squamipinnis were collected from the RAS system setup in the hatchery, where fishes were allowed to spawn naturally. Once fishes started courtship behaviour, a 200-µ mesh egg collector was kept below the pipe attached to the overflow mechanism. The egg collector was observed regularly for floating eggs. Fishes of the genus Pseudanthias are pelagic spawners, and these fishes do not exhibit any parental care after the spawning. The frequency of spawning varies with species, and in P. marcia, the fishes spawned on an average 25 days/month except for 5 days around the new moon (Anil et al., 2018).
Fish usually spawned between 18:00 h and 20:00 h in the late evening. The total number of eggs spawned per day ranged between 4,000 to 10,800 in Marcia’s anthias, and in sea goldie it was 200 to 5,000 (from a group of females). The fertilization rate of 50%–90% was observed in different rearing trials of P. marcia and P. squamipinnis.
Embryonic development and hatching
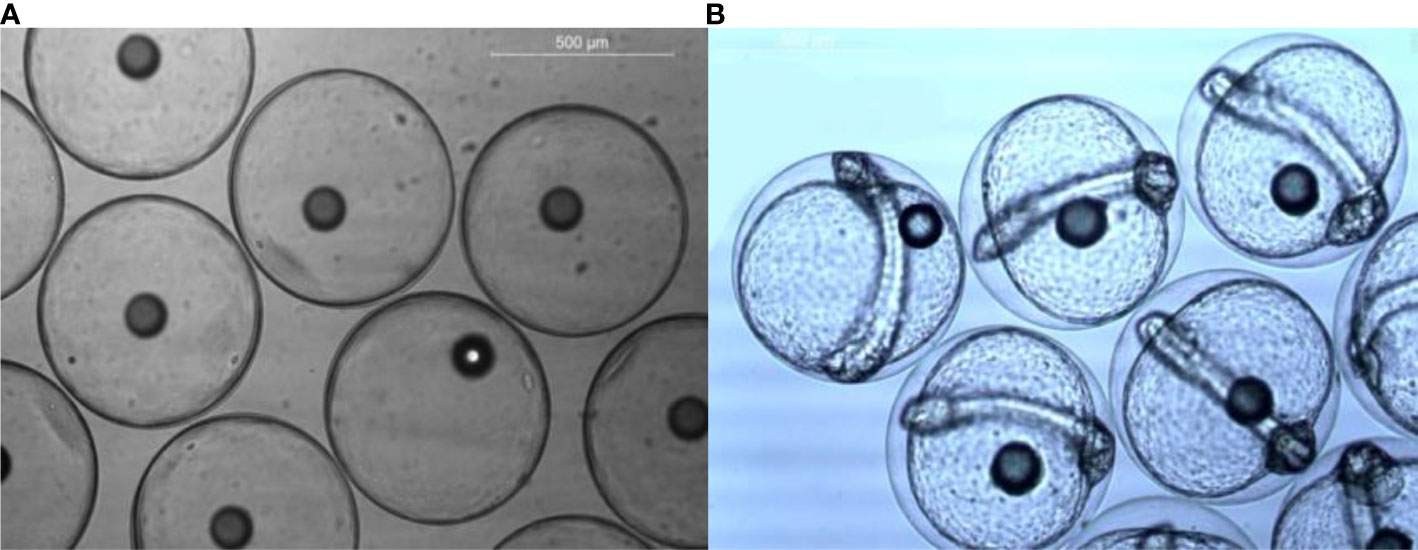
Fertilised eggs were planktonic, homogenous, spherical, non-adhesive, and transparent. The size of the individual eggs varied between 615 and 661 μ, and the average size was 617.891 ± 14.9 μ (mean ± SD) and had a single oil globule with a size range of 114 and 132 μ. Unfertilised eggs were opaque and settled to the bottom. Eleven hours post fertilization (PF), the eggs attained the optic vesicle stage of embryonic development and were collected from the broodstock tank for further stocking (Figure 7). The salinity and pH of filtered and pretreated seawater used for egg incubation were 35 ppt and 8.1, respectively, and 50% of the seawater was changed twice daily.
Larval rearing and feeding schedule
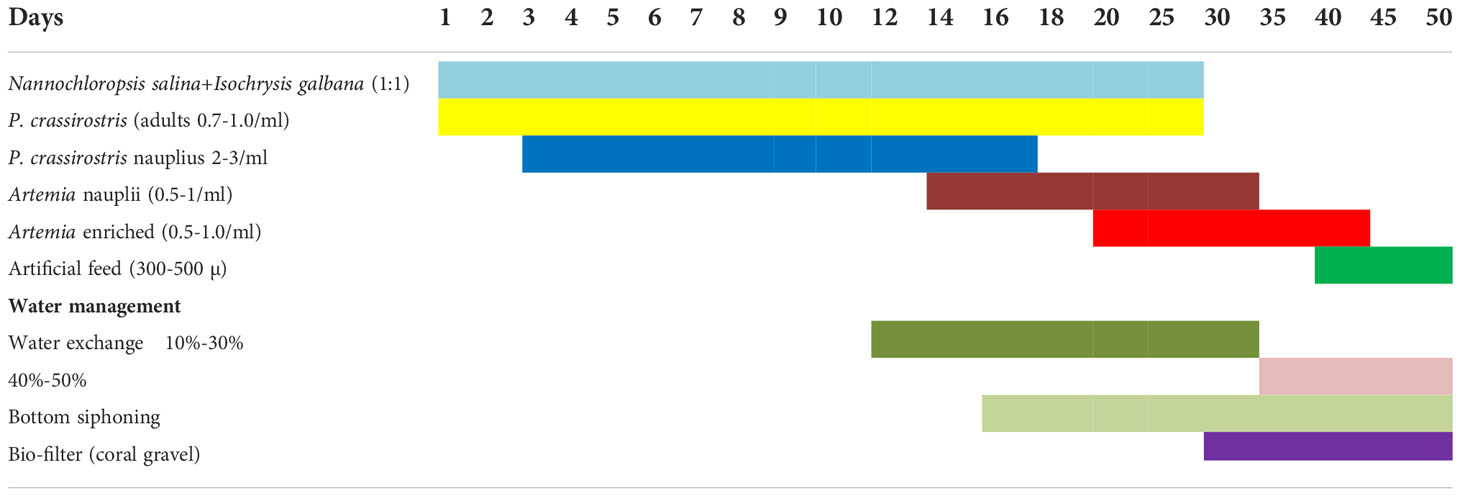
For larval rearing, eggs at the optic vesicle stage were collected from the overflow conduit of RAS and stocked in a 500-l FRP tank provided with green water medium and mild aeration. Green water medium was prepared by using a combination of algae such as N. salina and I. galbana (1:1 ratio). Water quality parameters of the larval rearing tanks were maintained at optimum level and monitored and recorded twice a day till larval metamorphosis. The collected eggs treated with Iodophor were transferred to the larval rearing tanks (10 eggs/litre in a green water medium) and hatched within 15–16 h post-incubation at 28 ± 1°C. The hatching rate of 78% to 85% was observed in different rearing trials of anthias species. Gomathi et al. (2020) studied the embryonic and larval development of P. marcia from hatchling to 30 dph and are given in Figure 8. The feeding and water management regime of Anthid fishes are given in Table 7.
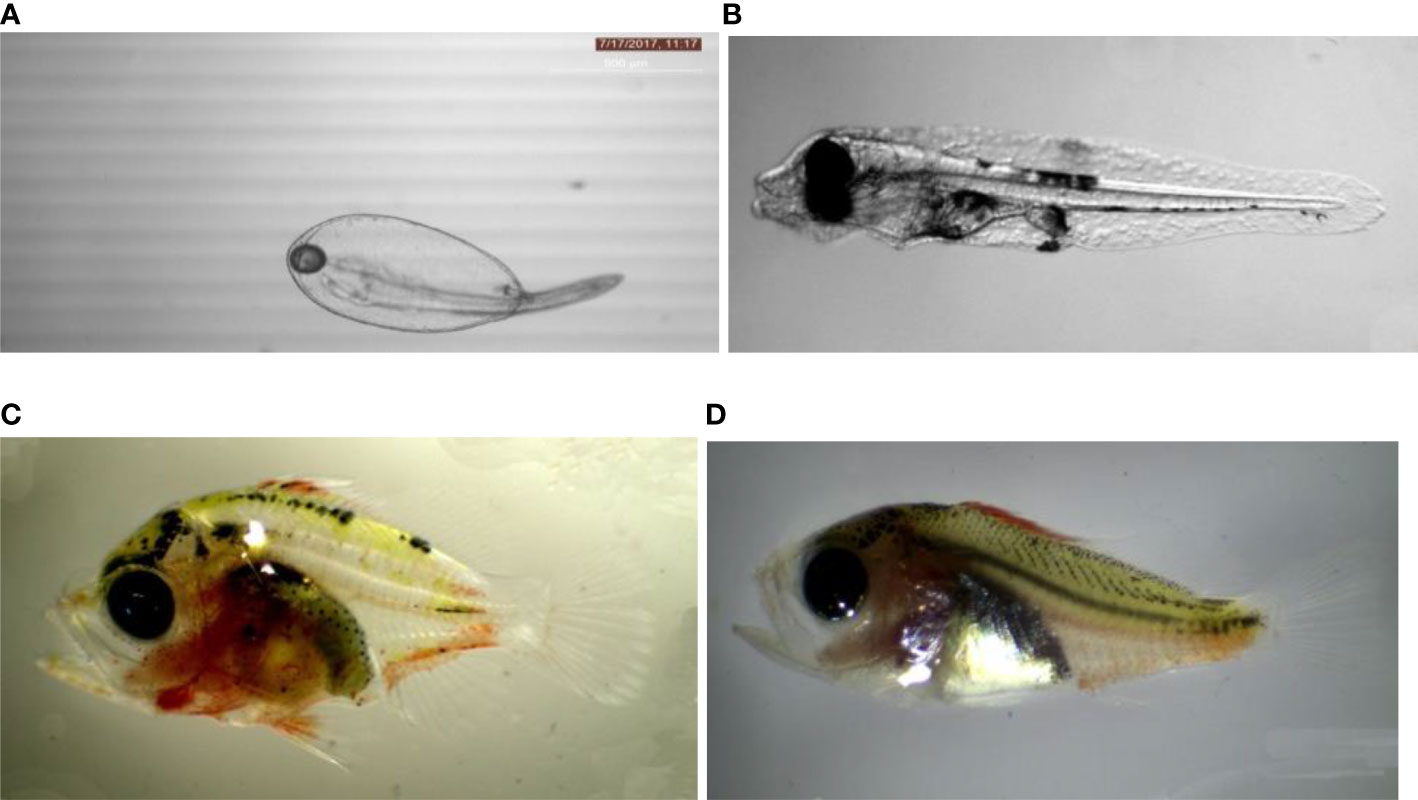
Figure 8 Larval development of Pseudanthias marcia from hatchling to 35 dph: (A) hatchling with yolk sac (B) 3 dph larvae, (C) 25 dph larvae, and (D) 35 dph larvae.
Fertilised eggs hatched within 14–16 h after spawning at a water temperature of 28 ± 1°C. The larvae came out of the egg membrane by twitching and wriggling movements. Yolk and the oil globules are the endogenous nutrient sources of the newly hatched larvae. Yolk-sac larva, characterised by a huge yolk sac (measuring 1,206.55 ± 100.02 µ) and single oil globule (measuring 120.39 ± 17.90 µ) located at the anterior-most tip of the yolk sac, were found floating in the surface layers and appeared transparent with unpigmented eyes and with the mouth and anus not formed. By 3rd dph, yolk sac absorption was completed and the mouth was open.
The first feeding of larvae began between 3 and 4 dph for copepod nauplii. On 10 dph, larvae swam vertically and were seen capturing artemia. By 25 dph, larvae were still planktonic, but they were in an advanced stage of development with a deep and transparent body.
Metamorphosis of the larvae began between 30 and 40 dph which was completed by 35 to 40 dph, during which the larvae became juvenile developing the adult colouration. The typical larval development of P. marcia is given in Figure 8.
In an improved larval rearing technique, larvae were fed with live feed till 40 dph to achieve a better survival rate. From 40 dph onward, juveniles were fed with an artificial diet of 300–500-µ size. Bottom waste was siphoned out from 12 to 35 dph at 4–5-day intervals. Standard operating procedures (SOPs) for ornamental fish breeding and larval rearing is detailed in Table 8.
Conclusion
Marine ornamental aquaculture is still in its infancy, and most of the fishes traded are from wild collections. There are only a few species whose breeding protocols have been standardised. Recent advances in RAS technology and advancements in life feed culture have helped to develop breeding technology for a good number of species, and there is every chance for this industry to develop into a multi-billion dollar sector. This hatchery-based technology helps us to formulate different strategies for the production of marketable adult fishes based on their growth rate, other developmental changes in larval and juvenile stages, and the changes in the colour and banding patterns of the abovementioned fishes. Global warming, climate change, and anthropogenic activities are threatening the very existence of the coral reefs worldwide and their inhabitants. Therefore, there is an urgent need to give proper attention to this sector as an alternate livelihood option for the fisherfolk along the seacoast and traders spread across the globe and conserve these delicate reef fishes.
Author contributions
MKA - Designing of RAS and Research ideas for broodstock development and larval rearing, manuscript preparation. MVRK - Research work on breeding of clownfish and damsels, manuscript preparation. PG - Research work on breeding of clownfish and anthias, manuscript preparation. SS - Research work on breeding of clownfish and manuscript preparation. APG - Broodstock collection BS - Breeding of damsels, copepod culture RS - Larval rearing of ornamental fishes VA - Larval rearing of ornamental fishes PMK - Larval rearing of ornamental fishes BR - Broodstock collection OS - Water quality analysis KM - Breeding of ornamental fishes AG - Research Management.
Funding
Research was carried out under different projects of ICAR-CMFRI.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ajith Kumar T., Balasubramanian T. (2009). Broodstock development, spawning and larval rearing of the false clown fish, Amphiprion ocellaris in captivity using estuarine water. Curr. Sci. 97, 1483–1486.
Alshuth S. R., Tucker J. W., Hateley J. (1998). Egg and larval development of laboratory reared sergeant major, Abudefduf saxatilis (Pisces: Pomacentridae). Bull. Mar. Sci. 62, 121–133.
Anil M. K., Gomathi P., Ambarish Gop P., Surya S., Siju R., Raju B. (2021). First successful breeding of lyretail anthias under captive condition. CMFRI Newslett. 170pp.
Anil M. K., Gomathi P., Raheem P. K., Raju B., Philipose K. K., Gopalakrishnan A. (2018). Captive broodstock development, breeding and seed production of anthid fish (family: Serranidae) marcia's anthias, Pseudanthias marcia in recirculation aquaculture system (RAS). Aquaculture 492, 265–272. doi: 10.1016/j.aquaculture.2018.03.043
Anil M. K., Kakati V. S., Ganga U., Zacharia S. (1999). Larval rearing of seahorse hippocampus kuda under laboratory conditions. Marine Fisheries Information Service, Technical and Extension Series. 162, 23–25.
Anil M. K., Santhosh. B., Jasmine S., Reenamole S., Unnikrishnan C., Anukumar. A. (2010). “Techniques for the mass production of two species of clown fish: Clown anemonefish amphiprion ocellaris cuvier 1830 and spine cheek anemonefish premnas biaculeatus (Bloch,1790),” in The proceedings of the national seminar on technology and trade prospects in ornamental aquaculture, fisheries research and extension centre. Ed. Felix S. (Chennai: Tamil Nadu Veterinary and Animal Sciences University) 96–102.
Anil M. K., Santhosh B., Prasad O., George R. M. (2012). Broodstock development and breeding of black-finned anemone fish Amphiprion nigripes Regan 1908 under captive conditions. Indian J. Fish. 59, 77–82.
Anzeer M., F and Aneesh K. S., Abraham, Mijo V., Darsana S., Santhosh B., et al. (2019). Breeding, early development and larval rearing of cloudy damsel, dascyllus carneus Fischer 1885. Aquaculture 505, 374–385. doi: 10.1016/j.aquaculture.2019.03.001
Arvedlund M., Mc Cormick M. I., Ainsworth T. (2000). Effects of photoperiod on growth of larvae and juveniles of the anemonefish amphiprion melanopus (Naga: ICLARM) 23, 19–23.
Dhaneesh K. V., Nanthini Devi K., Ajith Kumar T. T., Balasubramanian T., Kapila T. (2012). Breeding, embryonic development and salinity tolerance of skunk clownfish, amphiprion akallopisos. J. King Saud Univ. – Sci. 24, 201–209. doi: 10.1016/j.jksus.2011.03.005
Gomathi P., Anil M. K., Raheem P. K., Neethu Raj P., RohiniKrishna M. V., Ambarish Gop P., et al. (2020). Egg and larval development of serranid fish marcia’s anthias, Pseudanthias marcia (Subfamily: Anthiinae) spawned and reared under captive condition. Thalassas: Int. J. Mar. Sci. 36, 23–31. doi: 10.1007/s41208-020-00193-0
Gopakumar G., Rani M. G., Jasmine S. (1999). Breeding and larval rearing of the clownfish amphiprion chrysogaster. Marine Fisheries Information Service, Technical and Extension Series. 161. 8–11.
Gopakumar G., Santhosi I., Ramamurthy N. (2009). Breeding and larviculture of the sapphire devil damselfish Chrysiptera cyanea. J. Mar. Biol. Ass. India 51, 130–136.
Gopakumar G., Sreeraj T., Ajith Kumar T. T., Sukumaran T. N., Raju B., Unnikrishnan C., et al. (2002). Breeding and larval rearing of three species of damselfishes (family: Pomacentridae). Marine Fisheries Information Service, Technical and Extension Series. 171, 3–5.
Green E. (2003). “International trade in marine aquarium species: using the global marine aquarium database,” in Marine ornamental species: Collection, culture, and conservation. Eds. Cato J. C., Brown C. L. (Ames, Iowa: Iowa State Press) 31–47.
Ignatius B., Rathore G., Jagadis I., Kandasami D., Victor A. C. C. (2001). Spawning and larval rearing technique for tropical clown fish amphiprion sebae under captive conditions. J. Aquacult. Trop. 16, 241–249.
Madhu K., Rema M. (2010). Successful captive breeding and juvenile production of the tomato anemonefish, amphiprion frenatus. Marine Fisheries Information Service; Technical and Extension Series. 205, 1–3.
Madhu K., Rema M. (2011). Breeding and larval rearing techniques for protandric tropical clownfish amphiprion percula under captive condition. J. Aquacult. Tropics 26, 143–160.
Madhu K., Rema M. (2014). Captive spawning and embryonic development of marine ornamental purple firefish Nemateleotris decora (Randall & Allen, 1973). Aquaculture 424-42, 1–9. doi: 10.1016/j.aquaculture.2013.12.027
Madhu K., Rema M., Gopakumar G., Sasidharan C. S. (2006). Breeding, larval rearing and seed production of maroon clown premnas biaculeatus under captive conditions. Marine Fisheries Information Service, Technical and Extension Series. 190, 1–5.
Madhu K., Rema M., Retheesh T. (2012). Broodstock development, breeding, embryonic development and larviculture of spine-cheek anemonefish, Premnas biaculeatus (Bloch 1790). Indian J. Fish. 59, 65–75.
Madhu K., Rema M., Retheesh T. (2016). Spawning, embryonic development and larval culture of redhead dottyback Pseudochromis dilectus Lubbock, 1976 under captivity. Aquaculture 459, 73–83. doi: 10.1016/j.aquaculture.2016.03.017
Molina L., Segade A. (2012). Aquaculture as a potential support of marine aquarium fish trade sustainability. WIT Transactions on Ecology and the Environment. 148, 15–25. doi: 10.2495/RAV110021
Murphy B. F., Leis J. M., Kavanagh K. D. (2007). Larval development of the ambon damselfish pomacentrus amboinensis, with a summary of pomacentrid development. J. Fish. Biol. 71, 569–584. doi: 10.1111/j.1095-8649.2007.01524.x
Potthoff T., Kelley S., Saksena V., Moe M., Young F. (1987). Description of larval and juvenile yellowtail damselfish, Microspathodon chrysurus, pomacentridae, and their osteological development.Bull. Mar. Sci. 40, 330–375.
Raheem P. K., Rohini Krishna M. V., Surya. S., Gomathi P., Ambarish. P. G., Santhosh B., et al. (2021). Breeding, larval rearing and growth of black Amphiprion ocellaris (Cuvier 1830) under captivity. Indian J. Fish. 68, 60–69. doi: 10.21077/ijf.2021.68.2.102354-08
Rhyne A. L., Tlusty M. F., Schofield. P. J., Kaufman L., Morris J. A. Jr., Bruckner A. W. (2012). Revealing the appetite of the marine aquarium fish trade: the volume and biodiversity of fish imported into the united states. PLoS One 7, 35808. doi: 10.1371/journal.pone.0035808
Rohini Krishna M. V., Anil M. K., Gomathi P., Raheem P. K., Neethu Raj P. (2018). Studies on the broodstock production and larval rearing of coral demoiselle Neopomacentrus nemurus (Bleeker 1857). Aquacult. Res. 50 (2), 412–422.
Rohini Krishna M. V., Anil M. K., Neethu Raj P. (2018a). Larval development and growth of red saddleback anemonefish, Amphiprion ephippium, (Bloch 1790) under captive conditions. Indian J. Geo-Marine Sci. 47, 2421–2428.
Rohini Krishna M. V., Anil M. K., Neethu Raj P., Santhosh B. (2016). Seed production and growth of Neopomacentrus cyanomos (Bleeker 1856) in captivity. Indian J. Fish. 61 (3), 50–56. doi: 10.21077/ijf.2016.63.3.55058-06
Santhosh B., Ambarish P. G., Surya. S., Anil M. K., Muhammed Anzeer F., Aneesh K. S. (2021). Captive breeding and seed production of azure damsel. CMFRI Newslett. 170pp.
Thomas D., Prakash C., Gopakumar G. (2015). Spawning behaviour and embryonic development in the sebae anemonefish Amphiprion sebae (Bleeker 1853). Indian J. Fish. 62 (4), 58–65.
Thornhill D. J. (2012). Ecological impacts and practices of the coral reef wildlife trade. Defenders of Wildlife. 179.
Wabnitz C., Taylor M., Green E., Razak T. (2003). From ocean to aquarium UNEP (Cambridge, UK: World Conservation Monitoring Centre).
Keywords: RAS, ornamental fish breeding, clownfish, damsels, anthias
Citation: Anil MK, Rohini Krishna MV, Gomathi P, Surya S, Gop AP, Santhosh B, Siju R, Anand V, Krishnapriya PM, Shalini O, Raju B, Madhu K and Gopalakrishnan A (2022) Recent advances in marine ornamental breeding and seed production at Vizhinjam Regional Centre of CMFRI India. Front. Mar. Sci. 9:907568. doi: 10.3389/fmars.2022.907568
Received: 29 March 2022; Accepted: 30 August 2022;
Published: 09 December 2022.
Edited by:
Archana Sinha, Central Inland Fisheries Research Institute (ICAR), IndiaReviewed by:
Kailasam Muniyandi, Central Institute of Brackishwater Aquaculture (ICAR), IndiaShyne Anand P.S, Central Institute of Brackishwater Aquaculture (ICAR), India
Copyright © 2022 Anil, Rohini Krishna, Gomathi, Surya, Gop, Santhosh, Siju, Anand, Krishnapriya, Shalini, Raju, Madhu and Gopalakrishnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. K. Anil, mkanil65@gmail.com
 M. K. Anil
M. K. Anil M. V. Rohini Krishna
M. V. Rohini Krishna P. Gomathi
P. Gomathi S. Surya
S. Surya Ambarish P. Gop
Ambarish P. Gop B. Santhosh
B. Santhosh R. Siju1
R. Siju1  A. Gopalakrishnan
A. Gopalakrishnan