

This article provides a complete chemical and biological explanation for various factors affecting pH and KH in your aquarium. For a simplified version containing the important parts, please see The Basics of pH and KH in Your Aquarium.
Water is called a universal solvent because a lot of things can dissolve in it. Ions are some of those things and pH, KH, and GH are measurements of the important ones. While they may be small and invisible in your tank, the ions discussed in this lesson play a life-or-death role for your shrimp. Understanding them thereby prevents a ton of common problems that may otherwise harm your shrimp and/or wallet.
If you get nothing else out of this lesson, remember that having stable parameters is much more important than getting the “perfect” pH, KH, or GH, even if your tank is slightly outside the recommended ranges. DO NOT chase a specific value.
Because there is so much to go over, this lesson has been broken up into the following: what pH and KH are; how they affect your shrimp; how to test for them; and how to control them. Similar sections on GH will be included in the next lesson, which should be published in the coming weeks.
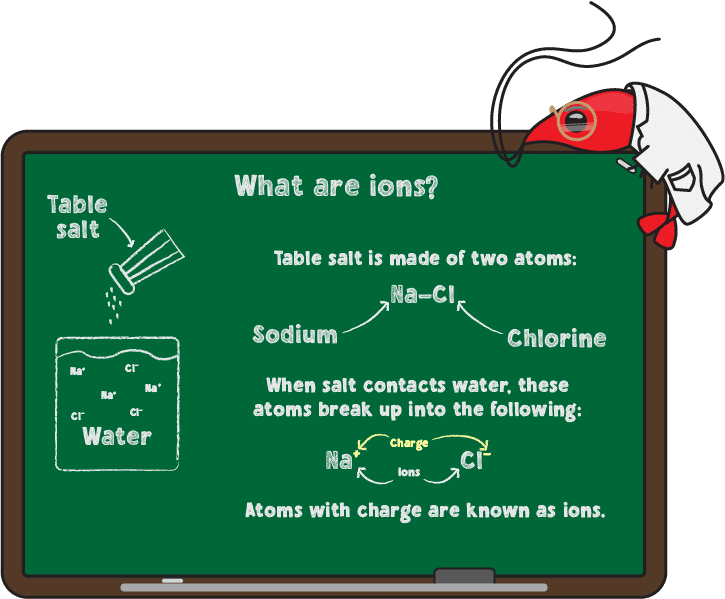
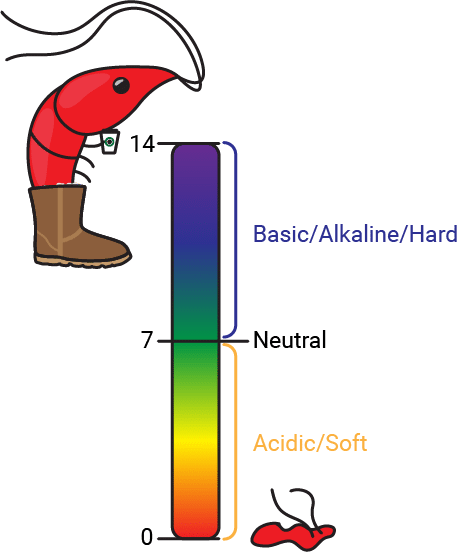
The potential of Hydrogen, or pH, is a measure of the concentration of hydrogen ions (H+) present in your water. As you may remember from nightmares about chemistry class, pH is measured on a scale of 0-14, with below 7 being acidic, at 7 being neutral, and above 7 being basic or alkaline. In terms of hydrogen ion concentration, 14 is the least concentrated and 0 is the most concentrated (although there are superacids much stronger than 0 pH). Acidic water is also referred to as soft water while alkaline water is called hard water.
Let’s go back to your chemistry nightmare of being melted alive in a tub of acid with the cackling of a fish witch drowning out your cries (C’mon, everyone’s been there). The reason that acids can melt through flesh is because they are extremely reactive, quickly giving hydrogen ions to many substances they come in contact with. The same is true of bases except the opposite reactions occur. Now, obviously, there should be nothing so extreme happening in your tank but the point is that many compounds in the water—and in the body of your shrimp—change as pH varies. Let’s look at ammonia as an example.
Ammonia can be found in two forms in your tank depending on the pH. At 7 pH or below, 99% of all ammonia is in a relatively non-toxic form. As pH increases, the percentage found in the toxic form increases, with approximately 5% taking on a toxic form at 8 pH, 10% at 8.4 pH, and 25% at 8.8 pH. So while you may not notice anything wrong if your tank has a low pH, a sudden increase in pH from a water change or chemical additive may create a stressful environment for your shrimp. That is why it is so important to make changes to the tank slowly.
For those interested in a slightly more scientific explanation, the non-toxic form is ammonium (NH4+), which occurs due to the higher concentration of H+ ions available in acidic conditions. This hydrogen is more likely to be lost in basic conditions, forming the toxic ammonia (NH3).
Shrimply Explained recommends a change of no more than 0.25 pH per hour.
Shrimp are extremely sensitive to these changes so their bodies experience stress which, if the shrimp are not killed outright, makes them more susceptible to diseases. Extreme pH changes are a common cause of fish and shrimp death, but even stable pH outside of each species limits causes problems, including but not limited to: stunted and slow growth, low reproduction rates, molting problems, diseases, loss of eggs, death, etc.
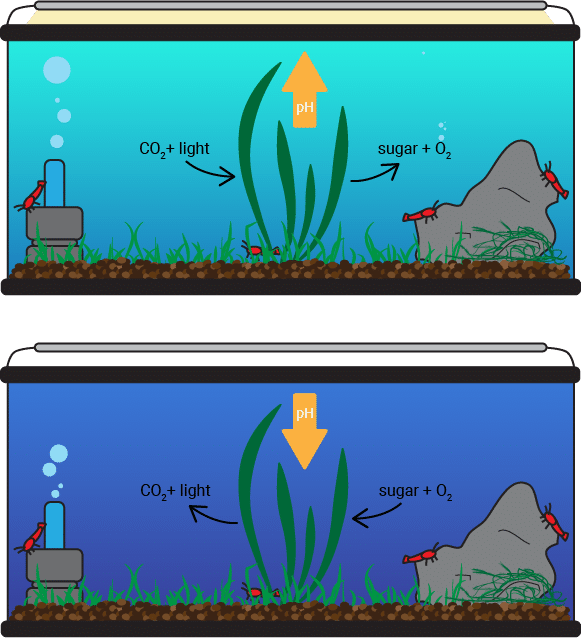
Two natural cycles in your tank cause most of the changes in pH – the nitrogen cycle and the photosynthesis cycle.
You are likely somewhat familiar with the nitrogen cycle if you’ve kept an aquarium before. It involves bacteria that transform ammonia (NH3) into nitrite (NO2-) and then nitrate (NO3-) and is the reason your tank must cycle before any animals can be added. These bacteria perform their job of breaking down ammonia best in the range of 7-8 pH and stop converting ammonia below 6 pH. Certain shrimp species prefer conditions at or below 6 pH, which leads to problems with ammonia build-up. This is part of the reason caridina shrimp like the Taiwan Bee are more difficult to care for.
While the nitrogen cycle is affected by pH, it also affects pH because the breakdown of ammonia into nitrite releases hydrogen ions. The reaction is shown below:
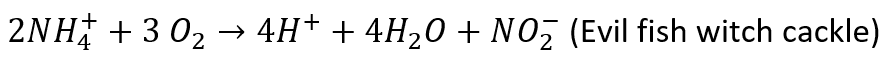
QUICK QUIZ: What happens to the pH when hydrogen ions are added?
ANSWER: It decreases.
If enough ammonia is being converted to nitrite, then there can be a significant drop in pH, which is why removing ammonia sources is so important (i.e., leftover food, dead or dying organisms, etc.). Adding live plants in the tank is another method of controlling ammonia and pH because they absorb ammonia before it goes through the nitrogen cycle.
The Photosynthesis Cycle
Aquarium plants turn light energy into usable chemical energy just like their above-ground brethren. This process—which allows plants and most life on earth to survive—is known as the photosynthesis cycle. A complex sequence of events occurs in photosystem 2 of a plant cell, followed by photosystem 1, and then some other stuff happens (Scientific as #$%!). I studied biology in college and all I remember is that the process had numbers out of order.
Anyway, here is what’s important for you to know:
During the day, plants uptake CO2, water, and light to produce sugar and release oxygen. CO2 is acidic, so its removal causes pH to rise gradually throughout the day until you turn the lights off. At night, plants perform respiration, intaking oxygen and using the produced sugar to grow while releasing CO2 and water. As a result, CO2 concentration and acidity increase throughout the night. If you were to measure the pH of your tank constantly for a 24-hour period, then you would see this gradual rise and fall.
If you have trouble remembering, just think of pH like the sun. They both rise in the morning and set (or fall) at night.
Even though there can be up to 1 full pH shift at the highs and lows of the photosynthetic cycle, the change happens so gradually that it does not stress out your shrimp. There may be a problem, however, if the average pH of your tank is already on the high or low end of the tolerable range, especially if there is a significant amount of ammonia in the water.
The cheapest and easiest way to test water parameters like pH (and KH) is with test strips. These are pieces of paper with squares of chemical-covered material attached that you dip into your tank for a couple of seconds. After a minute or so, the squares change color depending on the concentration of hydrogen ions in your tank and you compare the color to references on the bottle the strips came in. Because the exact amount of water that gets on each square of paper varies, these tests are not as accurate as other options.
The other option is using a test kit such as the one from API that requires you to take a sample of water from the tank and drip color-changing solutions into it. As with the test kits, you match the color of your sample with a reference provided in the test kit to determine pH. This is more expensive and may take longer than test strips, but the results are more accurate when performed correctly.
Lastly is a pH meter, which is an electronic device that provides a reading when you place it in your tank. This is the most accurate method and comparable in price to the test kits. The only issue is that pH meters become inaccurate over time and must be tested periodically with calibration solutions (often provided with the meter).
pH is closely tied to KH in your tank so the methods of control are the same. As such, these methods are combined into one section at the end of this lesson.
KH stands for carbonate (Karbonate) hardness and is a measurement of the amount of carbonate (CO32-) and bicarbonate (HCO3-) ions in your water. This water parameter is also referred to as total alkalinity. The units of measurement are either ppm (parts per million) when measuring with test strips, or dKH (degrees KH) with dropper tests such as the API KH Test Kit. If you ever need to convert between the two, then know that 1 dKH is equivalent to 17.6 ppm (rounding up to 20 may make it easier to remember though).
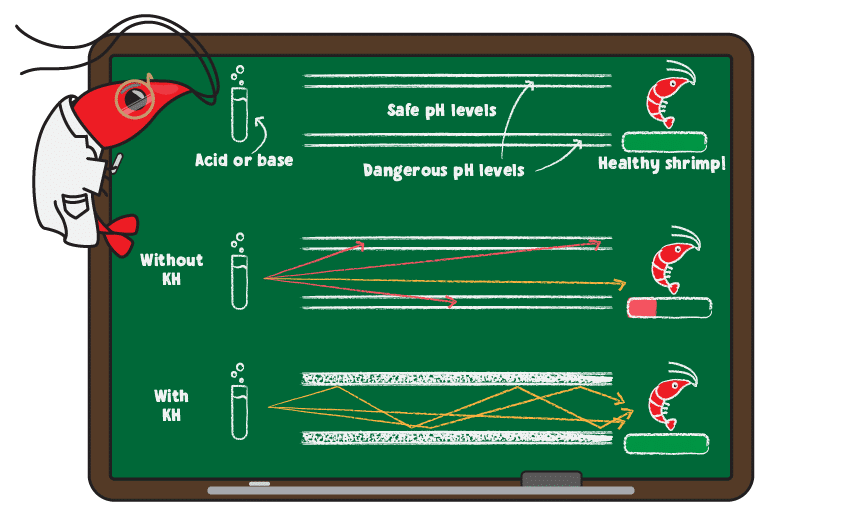
Carbonate and bicarbonate are responsible for maintaining a stable environment for your shrimp. They do so by absorbing acids and bases that enter the water, thereby minimizing pH changes. This is known as buffering in chemistry terms. A helpful analogy is to think of KH like the bumpers used in bowling alleys.
For anyone not familiar with bowling, the goal is to roll a ball down the lane (a narrow strip of smooth floor) to knock over as many pins as possible. On either side of the lane are gutters that the ball will fall into, directing the ball to the side of the pins and preventing you from getting points. For beginning bowlers, bowling alleys often block the gutters using bumpers, which are rubber bars the ball bounces off to keep it in the lane and to make scoring points easier. For this analogy, the ball is the addition of acid or base in your system, the lane represents safe pH levels while the gutters represent dangerous levels. Adding bumpers is the same as adding KH and scoring is the equivalent of achieving a healthy pH for your tank.
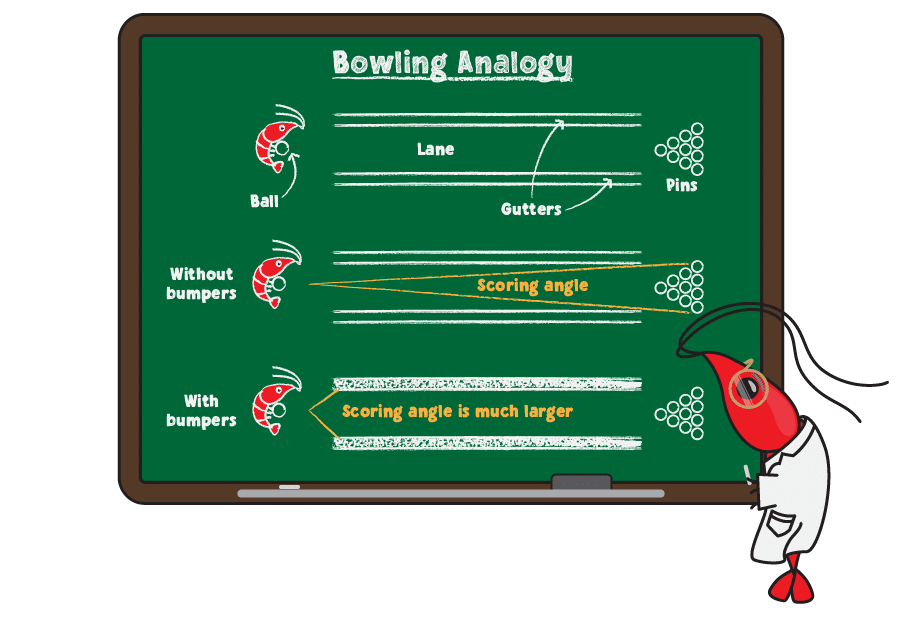
Without bumpers, there is a narrow range of angles that allow the ball to score. In contrast, bumpers widen the range of angles so that, even if the ball is rolled toward the bumpers, scoring is quite easy.
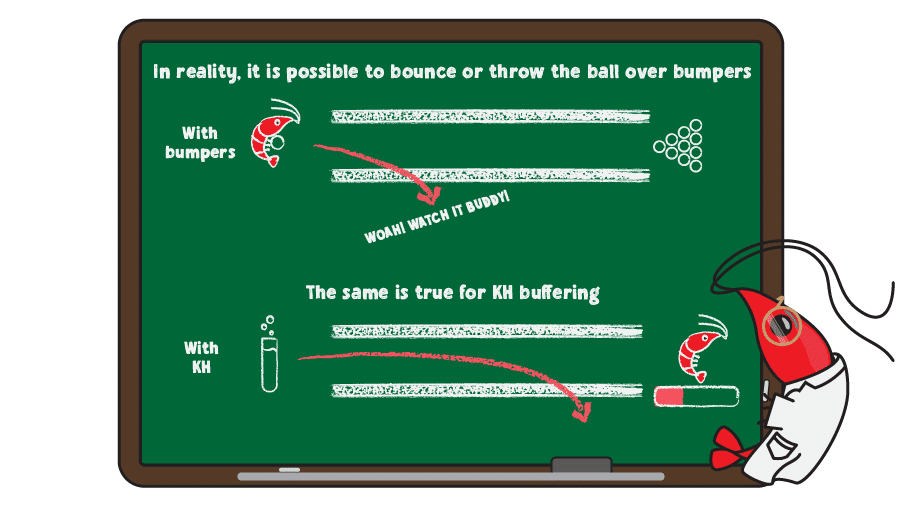
Even bumpers won’t allow every ball to score points though, as balls can bounce or be thrown over bumpers. This is true of KH as well, since too much of an acid or base overwhelms the buffering system, resulting in rapid pH change.
To make even the strongest, most uncoordinated person score in bowling, you could encase the entire lane in a cage of bumpers so that person can throw the ball in any direction, but that would be expensive and impractical for most bowling alleys. There is such a thing as too many bumpers.
The same is true of KH because, while buffering is great for preventing pH swings, too much KH causes high pH that is stressful for your shrimp. This is because carbonate and bicarbonate are basic compounds, meaning higher KH raises pH. Therefore, species that depend on high pH (Sulawesi shrimp) require higher KH, while caridina like blue bolts require 0-2 KH because pH would not be low enough otherwise.
KH decreases over time, partly due to nitrifying bacteria in your tank using carbonate to convert ammonia to nitrate. In addition, the buffering system gets used up if additional sources of acid are added to the tank.
For most shrimp keepers, all the information you need about KH has been provided above. The next short section is for those interested in learning more about the chemistry in your aquarium. It’s really cool because this is the same process that regulates the pH in your blood and in the oceans, but I won’t be offended if you skip it (Shrimply will be though).
As you know from the previous section on pH, changes in the concentration of hydrogen ions (H+) are responsible for shifts in pH. KH reduces these shifts by absorbing or releasing H+ through the carbonate/bicarbonate buffering system. The image below provides the chemical reactions involved.
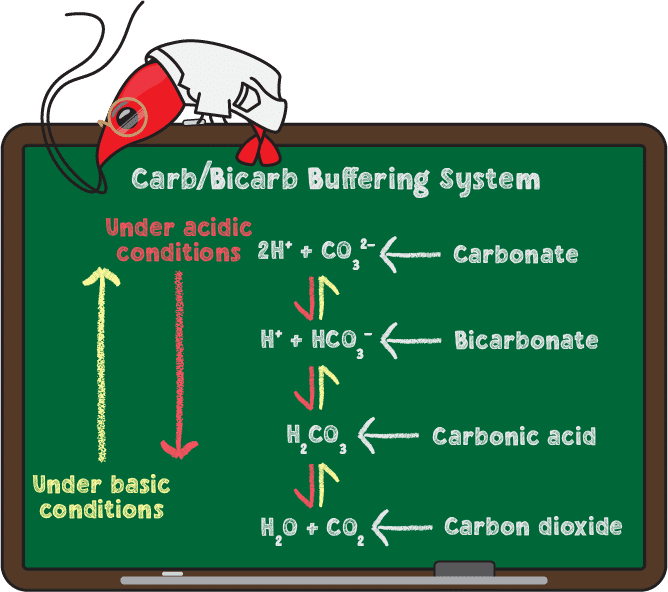
The arrows pointing both directions (↔) in the above equation signifies that these reactions are reversible. This means that when more H+ is present, carbonate converts into bicarbonate, then to carbonic acid, and finally to CO2 which gets released from your tank as gas bubbles. The loss of acidic carbon dioxide results in a higher pH. The opposite happens when there are low levels of hydrogen ions in the water.
Now you know a little more about what is occurring in your tank at a chemical level!
As we have established, KH and pH are related. This means that many common methods for lower KH also make your tank more acidic, and vice versa when KH is increased. Let’s go over some of the main methods of controlling KH:
1. Reverse osmosis or deionized (RO/DI) water
This water can be found at most grocery or fish stores and has been purified to remove almost all salts and minerals from it. As a result, the pH is just below 7 and KH is almost 0. Shrimp keepers that have alkaline tap water may mix in some RO/DI when setting up their tank and performing water changes. This lowers the pH and KH depending on how much the tap water is diluted. For example, if you have 12 KH tap water and want the water added to your tank to have 4 KH, then you need to mix 1/3 tap water with 2/3 RO/DI water.
Be aware that this water also has approximately 0 GH. More on that in the next section (Coming soon).
2. Buffering Substrate
Buffering substrate like Amazonia ADA Aquasoil and Fluval Stratum are acidic soils, meaning they release hydrogen ions into the water. This counteracts any basic/alkaline compounds in the water, including carbonate, thereby causing pH and KH to drop. These products typically lower and maintain the pH below 7. They do not affect GH.
Similar to KH, these substrates lose their buffering ability over time. As a result, they must be replaced periodically (every 6-24 months) to keep pH and KH low. Their buffering ability is also depleted faster if they are added to high pH/KH water, which is why soft water is recommended with these substrates. To avoid rescaping your entire tank each time new substrate needs to be added, you may put the substrate in an easy-to-remove container. This is not a very aesthetically pleasing solution, however.
Due to how expensive buffering substrates are and what a hassle they are to replace, I only recommend using them when keeping caridina shrimp that require low pH and KH. Some substrates also release ammonia initially so be careful when adding them to tanks that are already established.
3. Driftwood, Peat Moss, Chola Wood and Indian Almond Leaves (catappa leaves)
These natural materials all leach acidic compounds called tannins, which reduce KH and pH. Peat moss and Indian almond leaves also lower GH (more on that in the next section).
In addition to reducing pH, there is some scientific evidence that tannins also help protect your aquatic friends from diseases and improve healing after injuries. Dealing with shrimp disease is never fun, which is why I recommend having at least one item that releases tannins into your tank at all times.
One thing to be aware of is that your tank water will have a slight brown tint after adding any of these to your tank. This effect occurs because tannins are naturally dark and create a blackwater environment that is desired by certain breeds of fish. If you do not like the aesthetics of blackwater, then the wood/moss/leaves can be soaked in boiling water before adding them. Be aware that this results in fewer tannins released into your tank, thereby reducing the impact on pH, KH, and shrimp health.
Side Note: Peat Moss NOT Spaghnum Moss
While spaghnum moss and peat moss come from the same plant, their harvesting methods result in very different effects on water quality—so don’t use them interchangeably. Spaghnum moss is harvested directly from the living plant before being dried and used to maintain a moist environment for house or garden plants. In contrast, peat moss died, sunk to the bottom of a bog in Canada, then got mixed in with a bunch of other dead plant and animal matter before being harvested and dried. As a result, peat moss is far more acidic and provides additional nutrients to the water while spaghnum moss is of neutral pH and has no benefit to an aquarium (although it can be great for paludariums and terrariums). If you’re ever confused, just remember this rhyme:
Peat is neat.
Spaghnum is dumb.
Is it an unnecessarily aggressive saying toward regular spaghnum moss? Possibly - but that just makes it easier to remember.
4. Water Changes
If your local water is softer than your tank water somehow, then adding properly dechlorinated tap water to your tank is likely the cheapest way of lowering pH and KH. Keep in mind that, if you don’t normally use tap water for water changes, then you may introduce unwanted nitrates or metals (from rusted pipes) into your tank.
5. Acid buffers
Acid buffers are generally not something I recommend for two reasons:
1. Crushed Coral, Aragonite, and Dolomite
All of these are made of calcium carbonate and dissolve slowly in water to raise pH and KH (and GH). Each one can be added to the substrate or filter, but we recommend starting with a mesh bag in the filter so it is easily removable if you notice the pH rising too quickly. For the sake of safety, it is also best to start with a small amount, monitor parameters closely for a few days, then add or remove some after seeing the effects.
2. Water Changes
If your local water has higher pH and KH than your tank, then adding dechlorinated tap water may be a cheap solution. As I mentioned in Lowering pH and KH, this may introduce unwanted nitrates or metals (from rusted pipes) into your tank if you don’t normally use tap water.
3. Alkaline buffers
As with acid buffers, I am not a fan of these, but they have their uses in raising pH and KH specifically because ones like Continuum KH+ or Seachem Alkaline Regulator provide the only means of raising KH without affecting GH.
Whew, that was a lot to go over.
Now, you should have a good grasp on what pH and KH are, what role they play in your aquarium, how to measure them, and how to control them. With all that under your belt, you are one step closer to becoming a shrimp keeping master. If you still have questions, please reach out using the social media links at the top and bottom of the page, or send us an email at contact@shrimplyexplained.com. We are here to help however we can and love meeting new members of the community.
As mentioned previously, the next lesson is on GH and will be available soon. An email will be sent out once the lesson is online so sign up for our email list if you want to be the first to know!